Medical device manufacturer Medtronic is recalling certain HeartWare Ventricular Assist System (HVAD) heart pumps due to the possibility of an interruption in the electrical connection between the power source and HVAD controller. The U.S. Food and Drug Administration (FDA) is classifying this recall as a Class I, which is the...
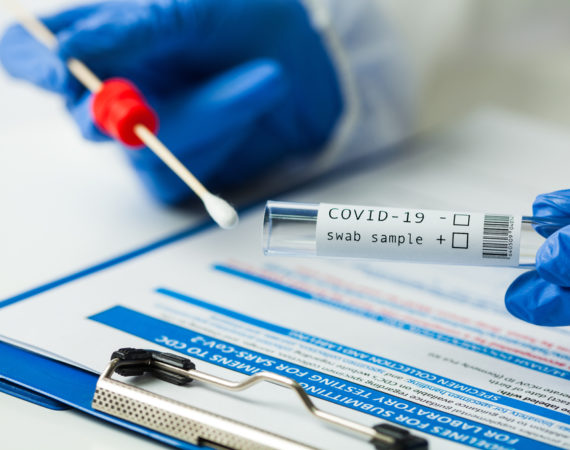
The Medical Devices Behind COVID-19 Testing
Whether surfing the internet, looking at Instagram or watching television, everywhere you look there is talk about COVID-19. A lot of the conversation about COVID-19 centers on the medical devices used for testing. From early on in the pandemic, medical device manufacturers have focused on better tests, better testing methods...
FDA Urges Consumers to Avoid Saniderm Hand Sanitizer due to Methanol Toxicity
Two distributors have agreed to recall Saniderm Advanced Hand Sanitizer at the guidance of the U.S. Food and Drug Administration (FDA). The product could contain a toxic chemical that is extremely dangerous to humans. More than a week before the recall notice, the FDA contacted Eskbiochem recommending the company pull...
FDA Announces More Metformin Recalls Due to Carcinogen
The U.S. Food and Drug Administration (FDA) has announced more Metformin recalls due to potential contamination with the carcinogen N-Nitrosodimethylamine (NDMA). The latest recall is the fifth involving extended-release Metformin tablets. For consumers who rely on Metformin to manage their Type 2 diabetes, Metformin recalls are reasonably concerning. Here is...
FDA Issues Class I Recall of Latex Balloon Catheters
The U.S. Food and Drug Administration (FDA) has designated a recall of Applied Medical latex balloon catheters as a Class I recall. This is the most serious medical device recall designation, meaning that there is a, “Reasonable probability that using the product will result in serious adverse health consequences or...
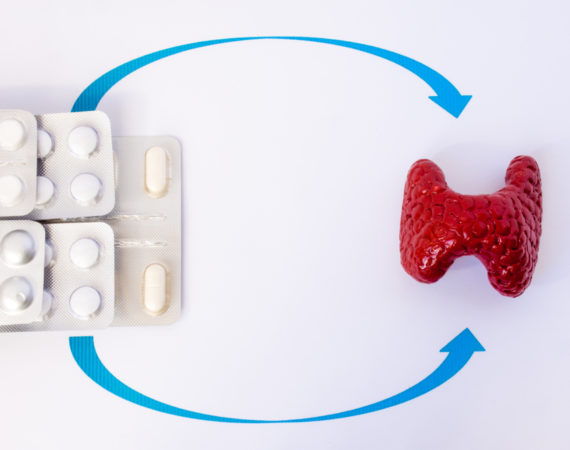
Thyroid Medication Recall: ‘Superpotent’ Hypothyroidism Drug Causes Hyperthyroidism
Acella Pharmaceuticals, LLC is voluntarily recalling certain lots of the hypothyroid medication NP Thyroid. According to the company announcement, the thyroid medication recall follows tests showing certain lots are “superpotent.” The affected products may have up to 115.0% more liothyronine (T3) than what is on the label. Read on to...
Talcum Powder Lawsuits: J&J Remove Talcum Powder from U.S. and Canada
Besieged by talcum powder lawsuits, Johnson & Johnson (J&J) has announced that they will discontinue all talcum-based baby powder products in the United States and Canada. This news is certainly a shift for the consumer product, drug and medical device giant. For many years, J&J has defended their talcum-based baby...
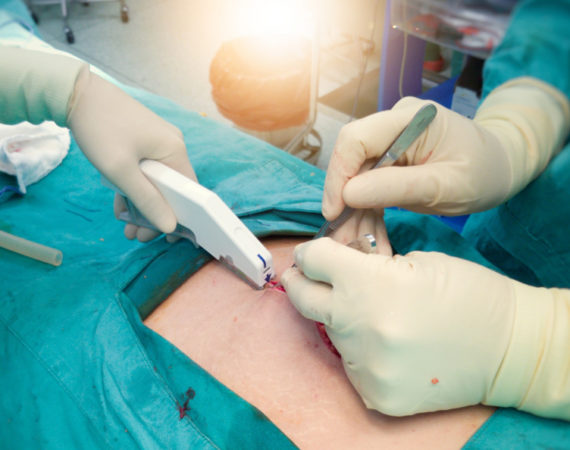
Lawsuits Surge Against Surgical Stapler Manufacturers
Lawsuits against surgical stapler manufacturers are surging, according to recent news. In 2019, surgical staplers made headlines due to recalls and adverse events reports that came to light after years of being hidden in a “secret database.” In June 2019, the U.S. Food and Drug Administration (FDA) released six million...
Another Heartburn Medication Recall Due to NDMA Concerns
Following on the heels of massive Zantac and other heartburn medication recalls, yet another medication is being recalled due to concerns about NDMA (N-Nitrosodimethylamine). The U.S. Food and Drug Administration (FDA) announced a recall of certain lots of Amneal Pharmaceuticals, LLC Nizatidine Oral Solution. Here is what we know about...
Boston Scientific Medical Device Recall Upgraded to Class I by FDA
In February 2020, Boston Scientific announced a recall of Imager II 5F Angiographic Catheters. The medical device recall came after the company reported receiving more complaints than usual about the device’s tip becoming detached. Now, the U.S. Food and Drug Administration (FDA) is upgrading the recall to a Class I,...
FDA Halts Inspections of Drugs and Medical Devices Manufactured Overseas
The U.S. Food and Drug Administration (FDA) has halted inspections of drugs and medical devices manufactured overseas. According to the FDA, the decision is due to the spread of the novel coronavirus, COVID-19. First, the FDA pulled back inspections in China, but has since pulled back from other countries, including...
Zantac Recall Update: FDA Calls for Removal of All Products from the Market
After months of investigating ranitidine products for the presence of a known human carcinogen, the U.S. Food and Drug Administration (FDA) is now calling for complete removal of all products containing ranitidine from the market. In January 2020, we wrote about the Zantac recall and worries about contamination with N-Nitrosodimethylamine...