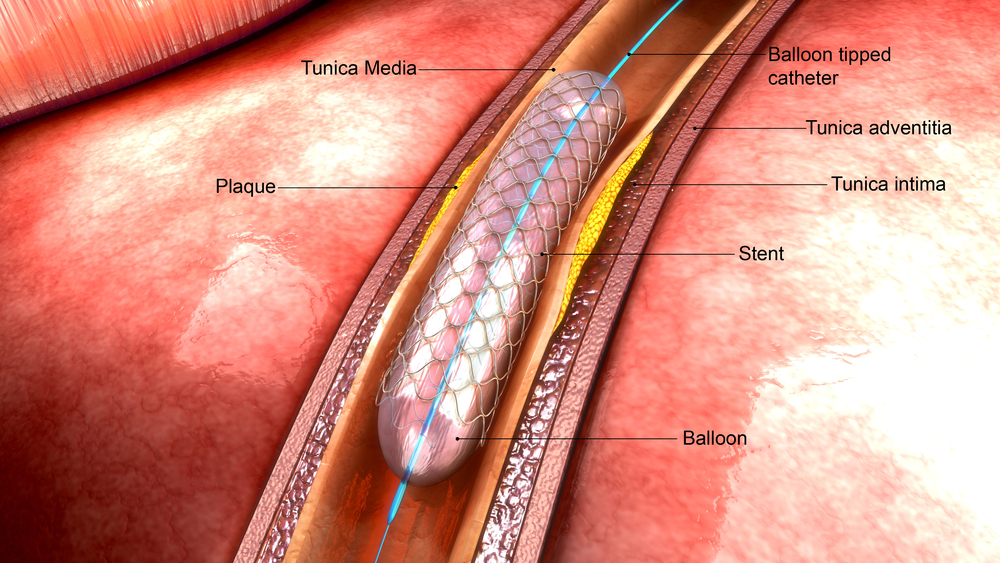
The Wingspan stent system manufactured by Stryker was recently the subject of a postmarket surveillance study. The results of the WEAVE study prompted an device safety alert from the U.S. Food and Drug Administration (FDA), which reminded healthcare providers about the dangers of using medical devices outside of their intended purpose.
The study, called Wingspan Stent System Postmarket Surveillance (WEAVE), analyzed data from 198 patients. The study’s findings suggested an increased risk of adverse events when using the device for off-label purposes, or purposes outside those approved.
The Danger of Off-Label Use
Medical devices regulated by the FDA have a strictly defined target group for whom their use would be appropriate. Use by physicians on patients outside the target population is called off-label use. Though manufacturers are prohibited by law from advertising or promoting their products for off-label use, individual physicians are within their rights to use discretion in using a medical device on a patient outside the target population. Physicians must consider the device to be the best treatment in their professional opinion.
The role of the FDA in this system is to monitor adverse outcomes involving medical devices. The FDA works to identify any alarming trends, reports of side effects, defects or recalls – especially those that arise from off-label use.
Device Safety Alert: Wingspan Stent System
Since its release in 2005, the Wingspan stent system has been embattled. Advocacy groups have petitioned against the sale of the stent system, which is intended to prevent recurring strokes in patients who have a history of stroke.
A stent works by propping open narrowed blood vessels in patients whose vessels are restricted from plaque buildup. When plaque builds up in the arteries of the brain, it is called intracranial stenosis or intracranial atherosclerotic disease. Those who suffer from it have a high risk of stroke.
Since the stent’s initial release, the FDA has issued a previous device safety alert narrowing the intended target population of the device to patients who meet ALL of the following criteria:
- Patients must be between the ages of 22 and 80 and have had two or more strokes despite aggressive medical treatment.
- Eligible patients must not have had a stroke within the seven days prior to treatment with the Wingspan stent.
- Patients must have 70-99 percent artery blockage due to atherosclerosis of the intracranial artery because of recurrent strokes, and have made a good recovery from their previous stroke.
- Patients must have a Rankin Scale score of 3 or less prior to treatment. Rankin Scores measure a patient’s degree of disability.
Off-label Use of the Wingspan Stent System
Prior to the FDA’s redefinition of the ideal target population of the device, there was a high rate of stroke and death among the patients treated with this device, hence the public outrage and petitions. Despite the new and more restrictive guidelines for use, off-label treatments have continued. That is why the FDA issued its most recent device safety alert. Stryker’s WEAVE study showed undeniable proof that off-label use of their stent is dangerous.
In their device safety alert, the FDA said the Wingspan stent system has a valuable benefit for patients who meet the criteria for the device. The federal agency intends to leave the device on the market with their approval for now. However, the FDA cautions physicians in no uncertain terms that off-label use for patients can be deadly.
The FDA has been aware of concern about this medical device for quite a while, which is why they asked Stryker to conduct a postmarket study. The results of the company’s WEAVE study are damning:
- They studied the outcomes of 198 patients who received the device .
- There were 152 patients identified as the ideal target population, 46 received off-label treatment.
- Four patients died within 72 hours of treatment, which was 1.3 percent of the target group, and 4.3 percent of the off-label group.
- Eleven total patients had a stroke within 72 hours of treatment, which was 1.3 percent of the target group, and 19.6 percent of the off-label group.
- Regarding patients who experienced no adverse outcome – 97.4 percent of the target group experienced neither stroke nor death, but only 76.1 percent of the off-label patents emerged unscathed.
- Every patient in the off-label group who suffered a subsequent stroke after the procedure experienced the stroke in the area of the stented artery. Seven of these strokes were ischemic, and two of them were hemorrhagic.
Is the Device Safety Alert Enough?
The consumer advocacy group who petitioned for the removal of the stent system from the market in 2011, Public Citizen, thinks the safety alert is not enough to protect American consumers. As a product of Public Citizen’s activism at that time, the FDA convened an advisory committee meeting regarding the stent system.
The panelists indicated that most patients did not have a significantly better outcome due to the stent device compared to patients who treated the same condition aggressively with medication. In other words, there is a specific population of patients with artery disease for whom this device is the most effective treatment option. Most others would be better served without the risks that the device and surgery present. It was on the advice of this panel that the FDA revised their recommendations about the patient population for this device.
Even with the WEAVE study findings, the advocacy group says the stent still doesn’t do enough for patients compared with the results patients get from medication treatment. More importantly, their spokesperson said, “You can’t have off-label use of a product that isn’t on the market, that’s the best way of stopping the off-label use.”
Understanding Stroke and Stent Use
The Stryker Wingspan stent system is a medical device used for the management of artery conditions that cause strokes. The strokes caused by artery disease that most often require stent treatment are called ischemic strokes.
An ischemic stroke is the sudden loss of blood circulation to an area of the brain, which results in corresponding loss of neurological function. Strokes can present with different symptoms depending on the area of the brain affected. The most common symptoms of a stroke are:
- Sudden paralysis one limb, one side of the body, or (rarely) all four limbs.
- Vision loss in one or both eyes.
- Loss of sensation, vision, or movement on one side of the body only.
- Facial droop
- Vertigo
- Ataxia, which is a marked decrease in awareness and clarity. Some physicians say the symptoms of ataxia mimic the symptoms of inebriation.
- Loss of ability to understand or express speech.
- Uncontrolled and repetitive motion of the eyes.
Minutes matter for successful recovery from a stroke. If you suspect someone you love is having a stroke, don’t stop to look up symptoms. Call 911 right away.
Need to Know More? Drug and Device Watch Can Help
Doctors have the option to use medical devices in an off-label manner because no federal regulatory agency can make a rule that will work for every single patient in every single circumstance. The mind of a physician familiar with a patient’s needs is usually the very best source of information on the most effective treatment for that patient as an individual.
That said, doctors have a responsibility to remain informed about developing device safety alerts and to consider FDA recommendations when they take on the responsibility of using a medical device for a purpose for which it was not originally intended. Sadly, sometimes doctors ignore warnings or risks, and use devices for off-label purposes despite what research and the FDA has to say. When that happens, they are negligently exposing the patient to adverse events.
If you or someone you love has had a traumatic medical experience due to the off-label use of a medical device, contact Drug and Device Watch at 1-888-458-6825 or submit our contact form online. We can help you understand and protect your rights as a patient and consumer.
Sources:
- https://www.medtechdive.com/news/fda-cautions-against-off-label-use-of-strykers-wingspan-stent-system/553553/
- https://www.mddionline.com/beware-label-use-wingspan-stent
- https://emedicine.medscape.com/article/1916852-overview
- https://www.fda.gov/news-events/fda-brief/fda-brief-fda-reminds-health-care-professionals-about-risks-wingspan-stent-system-after-study-shows