In September 2019, the ongoing blood pressure medication recall that has made headlines this year expanded once again. The U.S. Food and Drug Administration (FDA) announced that Torrent Pharmaceuticals Limited is expanding the recall of blood pressure medications at the consumer level.
According to the FDA, several batches of Losartan manufactured by Hetero Labs Limited were found to contain trace amounts of an impurity. The impurity is N-Methylnitrosobutyric acid (NMBA). The new recall includes only lots testing positive for NMBA above the FDA’s acceptable intake levels.
New Products in Blood Pressure Medication Recall
The FDA warns consumers to look at their medication label before taking Losartan products. If you already take the medication regularly, the FDA says to continue use until you talk to your doctor. Consumers can identify the products in the new recall by the following:
Losartan Potassium Tablets USP
- 13668-409-10 – 50mg, 1000 count, 4DU2E009, expires 12/31/2020
- 13668-115-90 – 100mg, 90 count, 4DU3E009, expires 12/31/2020
- 13668-115-10 – 100mg, 1000 count, 4DU3E018, expires 02/28/2021
Losartan Potassium/hydrochlorothiazide tablets USP
- 13668-116-90 – 50mg/12.5mg, 90 count, BEF7D051, expires 11/30/2020
- 13668-118-90 – 100mg/25mg, 90 count, 4P04D007, expires 07/31/2020
The FDA urges consumers to continue taking their medication. Consumers should consult with their healthcare provider about the risks of stopping use. Many people taking Losartan products affected by the blood pressure medication recall are now taking alternative blood pressure medications.
Why the Recall Continues to Expand
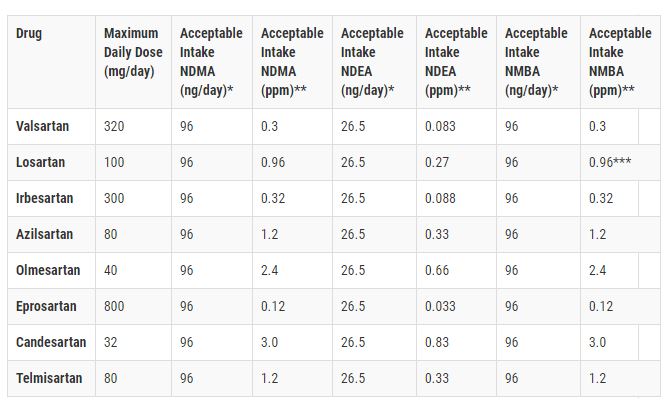
After months of recalls and five expansions, you may be wondering why the FDA continues to add to the list of Losartan medications in the recall. The original recall statement warns consumers about impurities found in Losartan products, namely N-Nitrosodiethylamine (NDEA) and N-nitrosodimethylamine (NDMA) and then N-Methylnitrosobutyric acid (NMBA).
Each of these impurities are probable human carcinogens per the International Agency for Research on Cancer (IARC). These substances occur naturally in some foods, water, air pollution, and as a result of industrial processes. No one seems to know where the impurity comes from in the manufacturing of the Losartan products.
What the FDA does know is that the products in the blood pressure medication recall contain these substances in levels higher than what they consider acceptable.
Prolonged exposure to NDEA, NDMA and NMBA can increase the risk of developing cancer. In laboratory studies, exposure to these substances caused cancer in rats. The rats were given higher doses of the potentially carcinogenic substances than what consumers are given in a dose of Losartan. Still, these substances can cause cancers of the bladder, lung, liver, and blood.
The FDA continues the blood pressure medication recall out of an abundance of caution.
Clearly, there is a problem somewhere in the manufacturing process of Losartan medications. While the recalls continue, the FDA continues to investigate the source of contamination. So many ongoing recalls suggest there is a systemic problem somewhere in manufacturing, monitoring, or shipping. Until the FDA can determine where the contamination is happening, consumers will continue to be at risk.
What Consumers Should Do with Recalled Blood Pressure Medication
If you take Losartan blood pressure medication and are concerned about the recall, the first thing you should do is contact your healthcare provider. Your doctor may advise you to keep taking Losartan despite the risks involved. Or, you may be given an alternative medication.
Torrent Pharmaceuticals Limited is working with Qualanex to have all affected lots of Losartan returned. Talk to your doctor or pharmacist to find out if you need to return your medication. If so, you may qualify for a refund.
If you experience any side effects from taking Losartan or have questions about the recall, you can contact Torrent Pharmaceuticals Limited by calling 1-800-912-9561. You can also report adverse events to the FDA via their MedWatch Adverse Event Reporting program.
If you have questions or concerns about your experience with the products in this blood pressure medication recall, you can also contact Drug and Device Watch. Our team of legal professionals can help make sure that your legal rights are protected, and you are treated fairly as a consumer. You can reach us by calling 1-888-458-6825, or fill out our online form to get started.
Sources:
- https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/updated-torrent-pharmaceuticals-limited-expands-voluntary-nationwide-recall-losartan-potassium-0
- https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/torrent-pharmaceuticals-limited-expands-voluntary-nationwide-recall-losartan-potassium-tablets-usp
- https://www.forbes.com/sites/brucelee/2019/03/03/what-new-impurity-is-causing-the-latest-blood-pressure-medication-recall/#16b82fbdefd3