Atrium Hernia Mesh Devices Recalled and Subject to a Federal Injunction
What should I watch out for?
A hernia occurs when an organ or fatty tissue squeezes through a weak spot in a surrounding muscle or connective tissue. There are many different types of hernias and many different treatments. One treatment for a hernia is surgery. Hernia surgery is very common in the United States, with about one million people undergoing the procedure yearly. In hernia surgery, doctors frequently use a hernia mesh device to repair the hernia. There are many different hernia mesh devices with many different manufacturers. Atrium is one of the hernia mesh device manufacturers. Atrium makes seven different hernia mesh devices. The seven devices are C-QUR, C-QUR Edge, C-QUR Mosaic, C-QUR Tacshield, C-QUR V-Patch, Proloop and VitaMesh Blue.
The Atrium hernia mesh devices were defectively designed and have caused serious and life-threatening complications to patients. Some of Atrium’s hernia mesh devices have even been recalled. There have been warnings from the United States Food and Drug Administration (“FDA”), lawsuits against Atrium for high infection rates and even a federal injunction to stop the production some of these hernia mesh devices. Despite all of this, Atrium hernia mesh devices remain on the market.
What side effects do defective hernia mesh devices cause?
Hernia mesh devices, including the seven Atrium hernia mesh devices, can cause the following side effects:
- Additional surgery
- Adhesion of tissue
- Bleeding
- Bowel and intestinal obstruction
- Hernia comes back after surgery
- Infections
- Movement of the mesh
- Pain
- Preformation of organs by the mesh
- Rejection of the mesh by the body
- Shrinkage of the mesh
If you receive a hernia mesh repair and have experienced any of the above-mentioned symptoms, contact your physician immediately.
Who makes hernia mesh devices?
Atrium Medial Corporation is a corporation that designs, develops, manufactures and distributes medical devices. It makes several products, including ventricular assist device components, endovascular devices, artificial heart components and hernia mesh devices. In 2011, Maquet Getinge Group bought Atrium Medical Corporation for $680 million.
Atrium’s hernia mesh devices are the C-QUR, Proloop and VitaMesh Blue. The C-QUR products have polyethylene plastic material and are coated with Omega-3 fatty acid fish oil. The C-QUR is intended for inguinal hernia repair and include the following models:
- C-QUR Mesh
- C-QUR Edge
- C-QUR Mosaic
- C-QUR Tacshield
- C-QUR V-Patch
The C-QUR mesh is unique because it has an absorbable coating derived from fish oil. Atrium used the fish oil to prevent inflammation and adhesions after hernia repair. However, studies have linked C-QUR mesh devices to increased adhesion and infection rates compared to other hernia mesh devices.
Similar to other hernia mesh devices on the market, Atrium’s C-QUR devices were approved by the FDA through its 510(k) process in 2006. These devices were allowed on the market because they were substantially similar to other hernia mesh devices on the market.
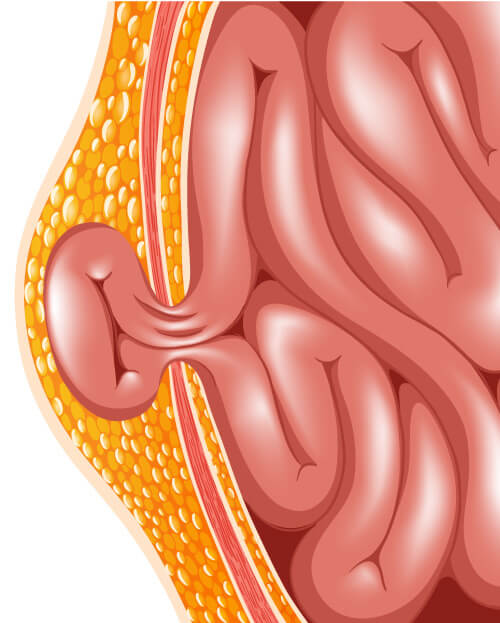
What is a hernia?
A hernia occurs when portions of the intestine or fatty tissue in the abdomen bulges out of the area where they are normally contained. A patient might not have symptoms of the hernia or might only have minimal pain. When the intestine or fatty tissue of some hernias bulge out of the abdominal wall, the blood supply to the tissue can cut off, making it a medical or surgical emergency.
Common types of abdominal wall hernias include:
- Inguinal (groin) hernia- are hernias that occur in the groin area where the skin of the thighs join the torso. Inguinal hernias occur more often in men than women and makes up 75% of all abdominal wall hernias.
- Femoral hernia- occurs in the middle of the upper leg. Femoral hernias occur more often in women.
- Umbilical hernia- are often seen in children at birth. An umbilical hernia occurs at the belly button and usually closes by the age of 2.
- Incisional hernia- occurs after abdominal surgery. Abdominal surgery causes a weakness in the abdominal wall, where a hernia can develop.
- Spigelian hernia- is a rare hernia that occurs along the edge of the rectus abdominus muscle.
- Epigastric hernia- occurs between the navel and the lower part of the rib cage.
- Hiatal hernia- occurs when the stomach pushes through the diaphragm.
- Diaphragmatic hernia- usually a birth defect causing an opening in the diaphragm, allowing a hernia to develop.
While abdominal hernias can be present at birth, other hernias develop later in life. Causes of abdominal hernias include:
- A family history of hernias
- Obesity
- Lifting heavy weights
- Coughing
- Straining during urination or a bowel movement
- Chronic lung disease
- Fluid in the abdominal cavity
Some hernias do not require treatment, while other hernias require surgery.
Tell me more about hernia mesh devices.
Approximately one million hernia repair surgeries are performed in the United States every year. There are many different hernia mesh devices manufactured by a multitude of companies. Current hernia mesh devices are made of either synthetic or animal tissue. Some devices are permanent fixtures in the body and others degrade in the body over time.
Throughout the years, surgeons have used surgical mesh to support the weakened or damaged tissues during hernia repair surgery. Hernia mesh devices are supposed to have the following benefits:
- Decrease surgical operating times
- Minimize recovery time for patients
- Improve a patient’s health by repairing the hernia
- Reduce the recurrence rate of anther hernia
Unfortunately, hernia mesh devices have caused serious complications in patients.
Did Atrium recall its hernia mesh devices?
In 2013, the FDA announced a class II recall of the C-QUR Edge mesh device. This recall affected over 145,000 units of C-QUR due to packaging problems. The recall was on Atrium devices C-QUR standard mesh devices, C-QUR V-Patch, TacShield, and Edge.
Atrium noted if the devices were exposed to excessive humidity over time, the humidity could “potentially cause the coating on the mesh to strongly adhere to the inner handling sleeve.”
Though Atrium warned doctors to examine the C-QUR mesh before implanting, Atrium did not remove any of the C-QUR devices from the shelf.
Did the FDA seek an injunction against Atrium?
As early as 2008, the FDA started receiving adverse event reports on the C-QUR products. The FDA inspected Atrium’s C-QUR manufacturing plants between 2009 and 2013 and discovered violations of federal regulations. The violations include manufacturing issues, as well as monitoring and reporting problems.
In 2012, the FDA sent Atrium a warning letter after the investigations. The warning letter revealed problems with manufacturing, sterilization, complaint follow-up and safety trials. The FDA noted Atrium employees failed to wear adequate sterilization clothing, including hair nets and disposable hats. Human hair and other foreign particles were found within the “sterile” C-QUR medical devices.
A 2013 FDA inspection turned up additional violations. On February 3, 2015, the FDA took stronger action by seeking an injunction against Atrium for ignoring repeated warnings about problems. An injunction is only given after multiple warnings are given.
The next day, the court agreed with the injunction, and issued a permanent injunction blocking the manufacturing and sales of C-QUR until Atrium fixed the problems.
While FDA injunction prevents the manufacture and sale of Atrium’s C-QUR products from its facility, it does not stop hospitals from implanting patients with the C-QUR hernia mesh. The C-QUR hernia mesh continues to be implanted in patients and is causing dangerous side effects.
Be on Watch
One of the many hernia mesh manufacturers is Atrium, which manufactures the following seven hernia mesh devices: C-QUR, C-QUR Edge, C-QUR Mosaic, C-QUR Tacshield, C-QUR V-Patch, Proloop and VitaMesh Blue. These devices have received FDA warnings, numerous complaints of high infection rates and even a federal injunction.
If you or a loved one had a hernia mesh repair and experienced symptoms, you may qualify for financial compensation for medical expenses, medical treatment, loss of income, and injuries suffered.
Call us at (888) 458-6825 or send us an email inquiry to discuss your legal options.
The consultation is free and confidential.
Resources:
- FDA Hernia Surgical Mesh Implants https://www.fda.gov/medicaldevices/productsandmedicalprocedures/implantsandprosthetics/herniasurgicalmesh/default.htm
- E Medicine Health https://www.emedicinehealth.com/hernia/article_em.htm
- FDA Class 2 Device Recall CQUR Mesh https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRES/res.cfm?id=119923
- FDA Class 2 Device Recall CQUR TacShield Mesh https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRES/res.cfm?id=119926
- FDA Class 2 Device Recall CQUR VPatch Mesh https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRES/res.cfm?id=119925
- FDA Class 2 Device Recall CQUR Edge Mesh https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRES/res.cfm?id=119924
- Cariovascular Business Mquet parent to buy Atrium https://www.cardiovascularbusiness.com/topics/coronary-intervention-surgery/maquet-parent-buy-atrium-680m
- S. Department of Justice injunction https://www.justice.gov/opa/pr/district-court-enters-permanent-injunction-against-new-hampshire-company-and-senior