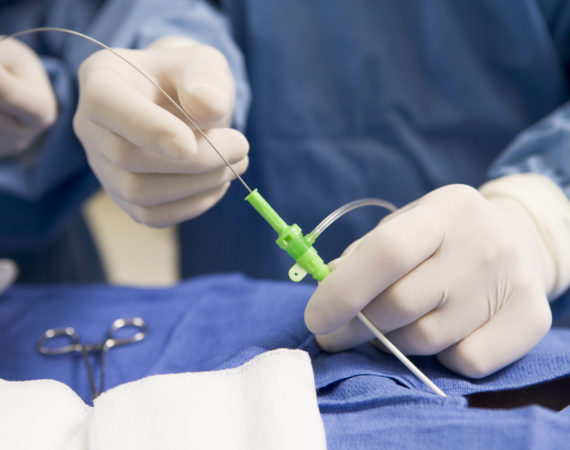
In July 2021, medical device manufacturer, Medtronic, initiated a device recall of some models of their Pipeline Flex embolization device. The Pipeline Flex device is used to treat brain aneurysms that balloon or bulge out the sides of affected blood vessels. The braided tube is a permanent cylinder used to...
Consumers Frustrated with Few Options Following CPAP Machine Recall
Consumers across the United States are frustrated as they find out there are few alternatives to their recalled CPAP machines. In June 2021, Philips and the U.S. Food and Drug Administration (FDA) announced a CPAP machine recall affecting millions of consumers. Now, consumers and their healthcare providers are scrambling and...
Philips CPAP Recall Due to Health Risks Affects Millions
Popular manufacturer, Philips Respironics, is recalling millions of CPAP (continuous positive airway pressure) and BiPAP (Bilevel Positive Airway Pressure) machines. The machines apparently contain foam that could be dangerous or could even cause cancer. Read on to learn more about the CPAP recall and how it could affect you if...
FDA Issues Class I Medical Device Recall for Medtronic Septostomy Catheters
The U.S. Food and Drug Administration (FDA) is categorizing Medtronic’s septostomy catheter recall as Class I. A Class I medical device recall is the most serious, meaning that use of the medical device could cause serious injuries or death. Here is what we know about the recall. Medical Device Recall...
Alaris System Infusion Pump Recall Includes 774,000 Units
Becton Dickinson (BD) CareFusion 303 Inc. is recalling certain lots of Alaris System Infusion Pumps due to system and software errors. The recall includes around 774,000 units. The U.S. Food and Drug Administration (FDA) has classified the recall as Class I, the most serious classification of recall. A Class I...
Medtronic Heart Pump Recall Follows Patient Death
Medical device manufacturer Medtronic is recalling certain HeartWare Ventricular Assist System (HVAD) heart pumps due to the possibility of an interruption in the electrical connection between the power source and HVAD controller. The U.S. Food and Drug Administration (FDA) is classifying this recall as a Class I, which is the...
FDA Issues Class I Recall of Latex Balloon Catheters
The U.S. Food and Drug Administration (FDA) has designated a recall of Applied Medical latex balloon catheters as a Class I recall. This is the most serious medical device recall designation, meaning that there is a, “Reasonable probability that using the product will result in serious adverse health consequences or...
Boston Scientific Medical Device Recall Upgraded to Class I by FDA
In February 2020, Boston Scientific announced a recall of Imager II 5F Angiographic Catheters. The medical device recall came after the company reported receiving more complaints than usual about the device’s tip becoming detached. Now, the U.S. Food and Drug Administration (FDA) is upgrading the recall to a Class I,...
FDA Announces Surgical Gown Recall for more than 9 Million Units
The U.S. Food and Drug Administration (FDA) is warning healthcare facilities to stop using certain surgical gowns made in China due to the possibility that they are not sterile. The recall issuer is medical device manufacturer Cardinal Health, but the gowns were made in China at a contractor facility. The...
Mavidon is Recalling All Medical Devices due to Burkholderia Cepacia Contamination
Mavidon – a large manufacturer of medical devices – is recalling all of their products worldwide due to possible contamination with Burkholderia cepacia. This worldwide medical device recall is making waves in the healthcare industry. Here is what we currently know about the recall. Worldwide Recall of Mavidon Medical Devices...
Women Face Risks and Decisions about Breast Implant Illness
Every year, millions of women undergo breast augmentation surgery. Most of these surgeries include installing breast implants - medical devices regulated by the U.S. Food and Drug Administration (FDA). Now, concerns about breast implant illness are causing many women to weigh the risks of implants and make difficult choices about...
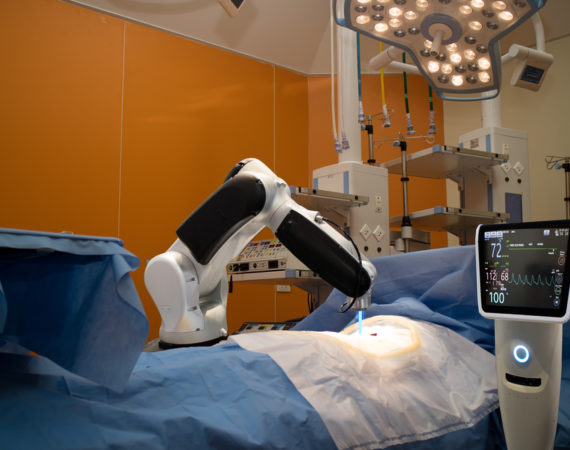
Zimmer Biomet Recalls ROSA Brain Surgical Robot Due to Software Problem
The U.S. Food and Drug Administration (FDA) has announced that Zimmer Biomet is recalling ROSA Brain 3.0 surgical robots due to a possible software defect. According to the recall announcement, there is a software problem that could cause the robotic arm to position itself incorrectly. The incorrect positioning of the...