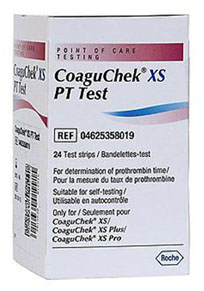
The U.S. Food & Drug Administration (FDA) reports that medical supply distributor Terrific Care/Medex Supply LLC has issued a voluntary device recall of certain test strips used to monitor patients who take the blood thinner warfarin. This FDA device recall has been classified as a Class I – the most serious classification of recall there is.
A Class I recall means continued use of the product in question could result in serious injury or death. Without swift action from the FDA, situations like this one can turn into widespread tragedy.
If you or a loved one uses warfarin with test strips, don’t delay. Contact Drug and Device Watch for a free consultation now.
FDA Device Recall Information
This FDA device recall is related to an FDA recall issued in November 2018 by the manufacturer Roche Diagnostics for these same CoaguChek brand test strips. The test strips, which are the subject of this recall, are also CoaguChek brand and also manufactured by Roche Diagnostics.
However, neither the manufacturer nor the FDA ever labeled or authorized these specific test strips for sale in the United States. Because they were never intended for sale in the U.S., the November 2018 recall did not include the current lot of test strips, which exempted them from the original recall and gave the false impression these test strips were safe to use.
The faulty strips are identical to the faulty ones from the first recall and should never have been available to U.S. consumers or healthcare providers.
It is of great concern to the FDA that U.S. consumers and healthcare providers were able to purchase these products. According to the recall notice, Terrific Care LLC /Medex Supply purchased these products intended for foreign markets from an unknown source and sold them in U.S. markets, creating a potentially deadly situation for consumers.
Which Devices are Being Recalled?
All CoaguChek XS PT Test Strips sold by Terrific Care/Medex LLC are being recalled. Identify the recalled devices by the following signs:
- Units manufactured between October 2017 to May 2018
- Units distributed in the US from December 27, 2017 to December 15, 2018
- Units distributed by or purchased from Terrific Care LLC/Medex Supply
- All lot numbers sold by Terrific Care LLC/Medex Supply
Dangers Associated with this FDA Device Recall
When a patient is on a powerful blood thinner like warfarin (sometimes prescribed under its brand names Coumadin and Jantoven), monitoring the dosage is critically important. That’s why at-home test strips are used to monitor a patient’s care in between office visits. Using test strips helps patients monitor the levels of warfarin in their blood, which determines what dose they should take to prevent blood clots.
When patients and physicians have inaccurate information about the effectiveness of a drug as powerful as warfarin, patients are in danger of serious harm or even death.
Though the distributor, Terrific Care LLC/Medex Supply, voluntarily issued this particular recall, this FDA device recall is ultimately taking place because the test strips do not function properly. It is based on the FDA’s original warning in November 2018, when it identified the danger of the CoaguChek XS PT Test Strips.
Using the manufacturer’s reports, the FDA determined that these at-home test strips simply give inaccurate results to the patient. These inaccurate results:
- Give patients and physicians inaccurate data about how long it takes the patient’s blood to clot
- Put patients’ lives in danger when warfarin dosages are changed based on this inaccurate data
- Put patients’ lives in danger when warfarin patients are wrongly instructed to stop taking warfarin
- Increase the risk of life-threatening blood clots in patients already at-risk for blood clots
Clearly, this product is not safe for consumers. If these CoaguChek XS PT Test Strips are affecting someone you love, call Drug and Device Watch today to find out more about your legal rights.
Who is at Risk?
Patients who take prescription blood thinners may have conditions ranging from:
- Existing blood clots in the lungs or legs
- Certain implanted medical devices like artificial heart valves
- Irregular heart rhythms
Blood thinners like warfarin are prescribed to millions of Americans each year. Achieving the correct dosage is absolutely crucial to the well-being of these patients. The potential loss of life and risk of serious injury due to these faulty test strips is tremendous.
What Should I do if This FDA Device Recall Affects my Family?
If you or a family member are taking warfarin and have used the recalled CoaguChek test strips, your first step should be contacting the prescribing healthcare provider. Stop using the strips immediately, and find out if your health has been put at risk. Your healthcare provider may be able to recommend another test strip you can use, and you can also contact the manufacturer of Coagucheck and get information about a refund or replacement.
Your next step should be contacting a drug and device attorney. Manufacturers and distributors have many legal responsibilities toward the consumers they market their products to. Products that are not approved for sale in the U.S. should never be a danger to consumers. Consumers who are harmed by dangerous medical devices like these test strips may be entitled to compensation.
What Consumers Can Do to Reduce Risk
While the FDA works hard to remove this product from American markets, consumers can participate in the following ways:
- Spread the word among family and friends about this dangerous product
- Inform your physician of this recall if you are blood-clot patient
- If this recall has potentially affected your healthcare, contact an attorney to learn about your legal rights
- Report any recalled test strips you happen to purchase in the future
You can also purchase replacement test strips directly from the manufacturer by calling 1-800-428-4674. If you have a product to return for reimbursement, you can contact the distributor over the phone at 888-443-2300 or by email at RMA@medexsupply.com
What if I Have Already Been Affected by This Recall?
If your treatment on blood thinners has been compromised by this medical device, your life may have been at risk. If blood clots are a daily concern for you, you don’t need to be told how dangerous it can be to take your medication improperly.
If your treatment on warfarin has been rendered ineffective due to these test strips, contact an attorney who is familiar with dangerous medical devices. At Drug and Device Watch, our attorneys can help review your situation and determine if you were one of the many people placed in harm’s way.
The goal of the FDA is to protect consumers from negligent and malicious action by manufacturers and distributors of medical devices and products intended for human consumption. When there is an FDA device recall, it means that consumers have been put at risk.
Contact a Device Safety Attorney
If you or a loved one has been harmed by products sold by Terrific Care LLC/Medex Supply, you may be entitled to compensation. An attorney knowledgeable about dangerous medical devices can help. Contact Drug and Device Watch by completing our online form or call today for a confidential consultation.