Integra LifeSciences and the U.S. Food and Drug Administration (FDA) are warning consumers about a recall of LimiTorr Volume Limiting Cerebrospinal Fluid (CSF) Drainage System and MoniTorr Intracranial Pressure (ICP) External CSF Drainage and Monitoring Systems. This FDA medical device recall is a Class I recall, meaning that using these medical devices presents a serious risk of injury or death.
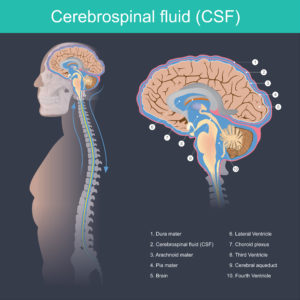
Medical professionals use the LimiTorr and MoniTorr devices to monitor pressure inside the skull and to drain cerebrospinal fluid (CSF) from the brain to reduce pressure.
Class I FDA Medical Device Recall
Integra LifeSciences announced their recall of the device in April 2019. In May, the FDA classified the recall as a Class I recall, which is the most urgent type of recall the agency monitors.
Integra LifeSciences announced the recall after receiving several reports of the device breaking or malfunctioning at the point where it connects to a transducer. Some of the reports of adverse events include:
- “piece breaking off at connection point”
- “broken manifold-stopcock”
- “stopcock problem”
- “connection where the external transducer is attached to the transducer holder bracket broke off”
- “transducer attachment on the LimiTorr/MoniTorr has cracked and was leaking CSF”
Because this device is a surgical tool, affected patients and their families may not realize the danger. In a medical emergency, brand names of medical devices are the last thing on patients’ minds.
For the medical professionals who use devices such as these every day, however, avoiding these malfunctioning devices can be the difference between a successful procedure and one that results in serious injury or death. Physicians and hospital administrators have a responsibility to remain informed about malfunctioning medical devices.
What are the Uses of this Defective Medical Device?
The drainage and monitoring systems subject to this FDA medical device recall are used to regulate and monitor CSF after brain surgery, major head trauma, or any neurological event that requires CSF monitoring for diagnosis. Specifically, the function of the devices is to:
- Relieve elevated intracranial pressure (ICP)
- Drain infected CSF
- Release and drain bloody CSF or blood after surgery or hemorrhage
- Monitor CSF, including flow rate
Injuries Associated with this Medical Device
The FDA reports that several patients have been seriously injured because of malfunctioning devices. The device can cause serious injury or death because the manner in which it malfunctions can allow too much CSF to escape, or may allow air into the brain cavity.
The adverse events associated with the devices in this FDA medical device recall may include:
Headaches
Not of particular concern alone, headaches after brain surgery can be an indication that something is wrong. In this instance, low CSF pressure due to the malfunctioning device is an early signal that more serious injuries can occur.
Fever
Especially if the device is intended to treat a patient with infected CSF, a sudden fever can be the indication that it is malfunctioning. Also, the introduction of air to the brain cavity opens the way to infection, the first sign of which is a fever.
Meningitis
The membranes around the brain and spinal cord are called meninges, and when these membranes are inflamed, the condition is called meningitis. Meningitis can develop due to bacterial or viral infection. The swelling from meningitis causes the telltale symptoms of a stiff neck, headache, and fever.
Ventriculitis
This condition describes the inflammation of the ventricles, or chambers, of the brain. Brain ventricles contain and circulate CSF throughout the brain. The malfunctioning medical device can cause this inflammation as a result of air or germs entering into brain tissue. This complication can be fatal without timely treatment.
Subdural Hematoma
Hematoma means “bruise, or a collection of blood under the skin.” A subdural hematoma is a condition in which blood collects between the layers of tissue surrounding the brain. The bleeding occurs between the outermost layer of tissues, the dura, and the next layer, called the arachnoid. The pooled blood can press on the brain and cause lasting damage or death.
Brain Herniation
This condition develops due to very high pressure within the skull. Herniation occurs when part of the brain is squeezed across structures within the skull. As a result, the brain can shift inside the skull. Likewise, the brain may protrude from the base of the skull where the spinal cord and brain meet. Herniation puts extreme pressure on parts of the brain and cuts off blood flow to the affected areas, meaning it is frequently fatal. It is so dangerous that hospitals and surgeons will take extreme measures to prevent herniation in an attempt to save a patient’s life.
Pneumocephalus
Pneumocephalus is the term that describes the presence of air in the cranial cavity. If the medical device allows air into the brain, it not only opens the way to infection, but also puts the patient at risk of a tension Pneumocephalus. This occurs when air compresses the frontal lobes of the brain and results in a distinctive malformation known as the Mount Fuji sign. This complication may be fatal.
Death
Medical devices that affect brain function or CSF levels are particularly dangerous. There is a serious risk of death as a result of using the LimiTorr Volume Limiting Cerebrospinal Fluid (CSF) Drainage System and its MoniTorr Intracranial Pressure (ICP) External CSF Drainage and Monitoring Systems.
What to Do if this FDA Medical Device Recall Affects You
If you or someone you love has experienced adverse side effects from the Integra LifeScience products in this recall, it is advisable to contact an attorney. At Drug and Device Watch, our attorneys offer legal advice for patients suffering as a result of a defective or dangerous medical device. Many patients have grounds to take legal action against the manufacturer if a product has caused them harm.
To find out more about the CSF drainage and monitoring systems in this FDA medical device recall, call Drug and Device Watch. We can certainly help you understand your legal rights and determine if you have an actionable claim. You can request a free consultation by calling 1-888-458-6825. You can also contact us online.
Source: