Following on the heels of massive Zantac and other heartburn medication recalls, yet another medication is being recalled due to concerns about NDMA (N-Nitrosodimethylamine). The U.S. Food and Drug Administration (FDA) announced a recall of certain lots of Amneal Pharmaceuticals, LLC Nizatidine Oral Solution. Here is what we know about...
In The News
Boston Scientific Medical Device Recall Upgraded to Class I by FDA
In February 2020, Boston Scientific announced a recall of Imager II 5F Angiographic Catheters. The medical device recall came after the company reported receiving more complaints than usual about the device’s tip becoming detached. Now, the U.S. Food and Drug Administration (FDA) is upgrading the recall to a Class I,...
FDA Halts Inspections of Drugs and Medical Devices Manufactured Overseas
The U.S. Food and Drug Administration (FDA) has halted inspections of drugs and medical devices manufactured overseas. According to the FDA, the decision is due to the spread of the novel coronavirus, COVID-19. First, the FDA pulled back inspections in China, but has since pulled back from other countries, including...
Zantac Recall Update: FDA Calls for Removal of All Products from the Market
After months of investigating ranitidine products for the presence of a known human carcinogen, the U.S. Food and Drug Administration (FDA) is now calling for complete removal of all products containing ranitidine from the market. In January 2020, we wrote about the Zantac recall and worries about contamination with N-Nitrosodimethylamine...
Roche Coronavirus Test Receives Emergency Use Authorization from the FDA
Medical device manufacturer, Roche Diagnostics, has received emergency use approval (EUA) from the U.S. Food and Drug Administration (FDA) for their Cobas SARS-CoV-2 coronavirus test. The test detects the virus COVID-19 through nasopharyngeal or oropharyngeal swab samples. The test can be used in patients who meet clinical or epidemiological testing...
Drug Manufacturers Developing Vaccines and Treatments for Coronavirus
The number of coronavirus (COVID-19) cases in the United States continues to rise. As more communities suffer infection, quarantines and general panic, researchers continue to learn whatever they can about how the virus spreads, who is most at risk and how the virus could be prevented. Drug manufacturers are among...
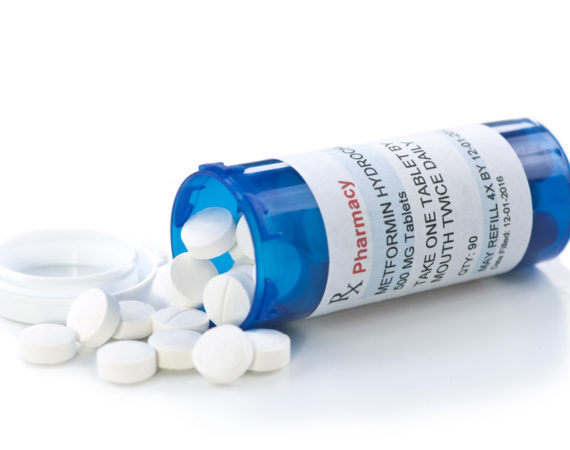
FDA Urged to Recall Metformin after Third Party Testing Shows NDMA in 16 Batches
Pharmaceutical companies are under more pressure than ever due to concerns about contamination and the presence of unsafe ingredients. Readers will no doubt recall notable recent medication recalls like Zantac (ranitidine), Valsartan and Belviq. Now, another popular drug, Metformin, is joining those ranks after third party tests found the probable...
Weight Loss Drug Belviq Recall: Drug Linked to ‘Increased Occurrence of Cancer’
Popular weight loss drug Belviq is being recalled after a safety clinical trial showed an “increased occurrence of cancer.” Following the trial, the U.S. Food and Drug Administration (FDA) requested a Belviq recall from Eisai Co., the manufacturer. Eisai acceded to the FDA’s request, though the company claims that their...
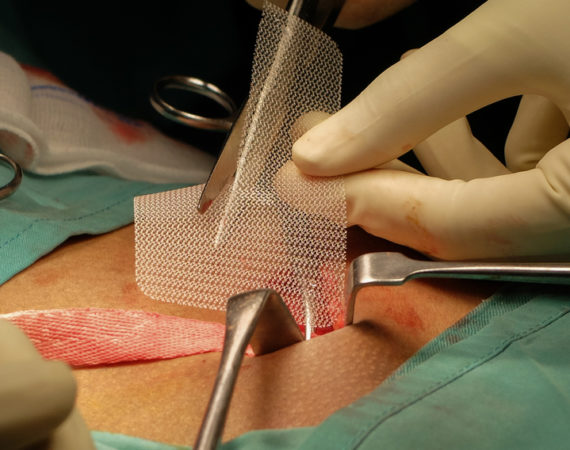
Trial Dates Set for Bard Hernia Mesh Cases
After years of moving through the legal process, the first trial dates are set for consolidated Bard hernia mesh cases. In January 2020, plaintiffs and defendants each selected the cases they wanted to proceed to trial. The court will choose three cases to proceed to bellwether trials. Once case-specific motions...
FDA Announces Surgical Gown Recall for more than 9 Million Units
The U.S. Food and Drug Administration (FDA) is warning healthcare facilities to stop using certain surgical gowns made in China due to the possibility that they are not sterile. The recall issuer is medical device manufacturer Cardinal Health, but the gowns were made in China at a contractor facility. The...
Cybersecurity Warnings Issued for GE Healthcare Medical Devices
The U.S. Food and Drug Administration (FDA) has issued a device safety alert for GE Healthcare medical devices. The safety alert relates to GE Healthcare Clinical Information Central Stations and Telemetry Servers. The FDA says that cybersecurity threats could risk the health of the patients that these medical devices monitor...
More Antacid Recalls Issued due to Trace Elements of NDMA
On the heels of notable recent medication recalls like Zantac, the U.S. Food and Drug Administration (FDA) is recalling even more medications due to possible trace elements of NDMA. NDMA (Nitrosodimethylamine) is a probable human carcinogen, meaning it may cause cancer. The presence of NDMA in Zantac has made waves...