The U.S. Food and Drug Administration (FDA) has announced a recall of more than 106,000 Medtronic heart catheters. According to the FDA announcement, the device recall follows numerous complaints about the devices not performing as expected. The FDA classifies this recall as a Class I. Class I medical device recalls are the most serious. A Class I means,
“Use of the medical device may cause serious injuries or death.”
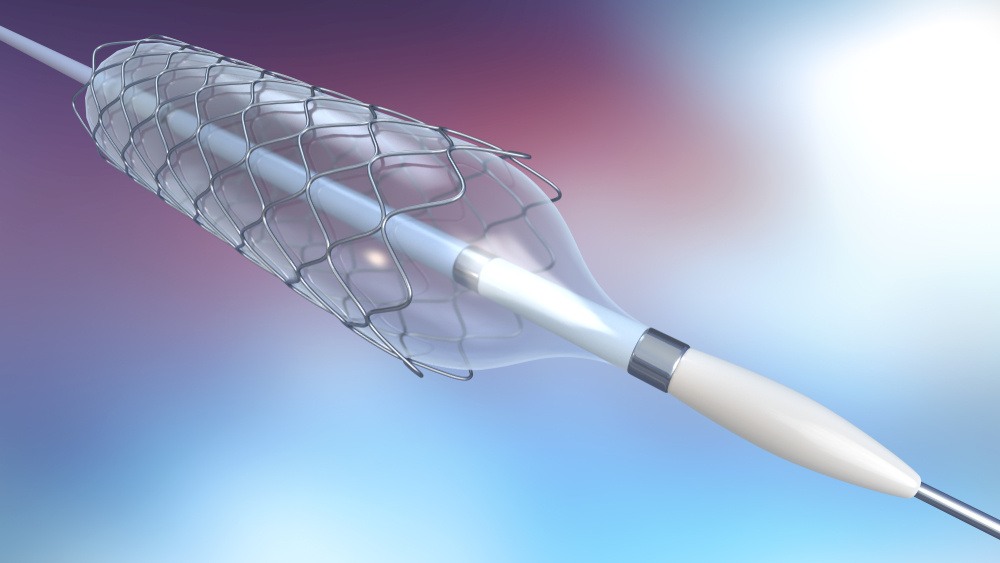
The device included in the recall is the 6 French Sherpa NX Active Guide Catheter.
What is the 6 French Sherpa Heart Catheter?
The Medtronic 6 French Sherpa NX Active Guide Catheter is used by healthcare providers to gain access to arteries and veins. It is used in procedures both inside and outside of the heart. The device assists healthcare providers when placing or exchanging guidewires and other devices, as well as administering fluids or drugs directly into a blood vessel.
Why is the French Sherpa Heart Catheter Being Recalled?
The 6 French Sherpa NX Active Guide Catheter is subject to this recall due to the possibility that the outer material could separate from the device. If this happens, detached fragments could remain inside a patient’s blood vessel. Fragments left inside a blood vessel are certainly dangerous, as are attempts to remove them. Possible complications include:
- Blockage of the blood vessel
- Injury to the blood vessel wall
- Blood clots
- Embolism
- Heart attack
- Death
The FDA reports at least five adverse events complaints related to the device. However, the company has not detailed the nature or severity of the injuries.
Information About Device Recall
Patients and healthcare providers can identify the heart catheters in this recall by the following:
- Name: 6 French Sherpa NX Active Guide Catheter
- Model: All models
- Manufacturing Dates: March 2017 to March 2019
- Number of Devices Recalled: 106,298
Healthcare providers are the primary users of these heart catheters, but patients should also be aware of possible exposure to a dangerous medical device. Medtronic requests that healthcare providers remove any 6 French Sherpa NX Active Guide Catheters from their inventory.
Who is Affected by the Medtronic Heart Catheter Device Recall?
This recall affects any patient who has undergone a procedure using the 6 French Sherpa NX Active Guide Catheter. Even though Medtronic is pulling the devices from use, many patients are already at risk of exposure to the potentially defective devices.
If you have undergone any procedure using these catheters, contact your healthcare provider to find out if you may be at risk. You can also learn more about the recall by calling Medtronic Cardiovascular Patient Services at 1-877-526-7890.
If you have been exposed to the 6 French Sherpa NX Active Guide Catheter and have suffered an injury related to the device, you are urged to file a complaint through the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
Should I Contact a Medical Device Lawyer?
As a patient, you have the right to know when you are at risk due to a dangerous or defective medical device. You also have the right to explore your legal rights if you have been injured by a medical device. If you have suffered an injury due to the 6 French Sherpa NX Active Guide Catheter, you should certainly contact a lawyer. You may have a claim for compensation.
At Drug and Device Watch, our medical device lawyers care about your health and legal rights. We can help you investigate the cause of your injuries and determine if you have an actionable claim. If so, we will help you build a claim in pursuit of justice and compensation.
To learn more about your legal rights or this Medtronic device recall, call Drug and Device Watch at 1-888-458-6825. You can also request a free legal consultation by completing our online contact form.
Sources: