The U.S. Food and Drug Administration (FDA) issued a warning early in June, 2019 about an ingredient in dietary supplements that can be dangerous for women of childbearing age. The FDA warning concerns the compound vinpocetine. Vinpocetine is an ingredient in supplements that increases cognitive performance, enhances energy, and reduces...
Details of a Medical Device Recall Kept Secret by the FDA’s Secret Reporting Pathway
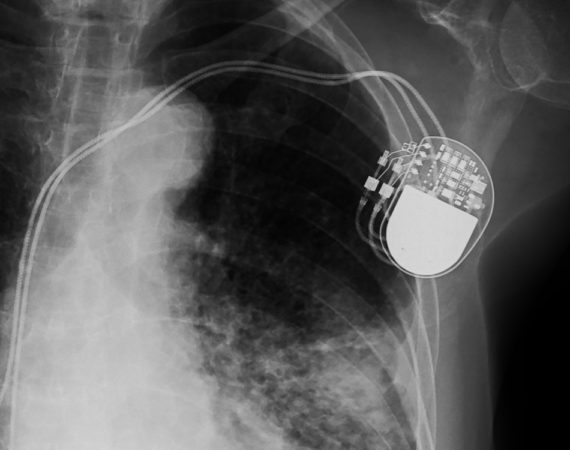
The details of a medical device recall involving an implantable defibrillator is alarming to say the least. The Sprint Fidelis, an internal defibrillator, is one of an unknown number of malfunctioning medical devices the U.S. Food and Drug Administration (FDA) kept hidden from the public via a confidential adverse event...
Former FDA Inspector Speaks Out About Blood Pressure Medication Recall
In a blow to the integrity of the U.S. Food and Drug Administration (FDA), a one-time inspector raised concerns about the agency’s recent and sweeping blood pressure medication recall. The former inspector, Massoud Motamed, suggests the FDA does not have the capacity to effectively monitor imported pharmaceuticals and that this...
Can Hackers Hijack Medical Devices?
Patients who rely on medical devices now have something more to worry about than just device malfunctions or defects. In today’s digital age, patients now have to worry about hackers compromising their healthcare and safety. At Drug and Device Watch, our goal is to make sure that patients and consumers...
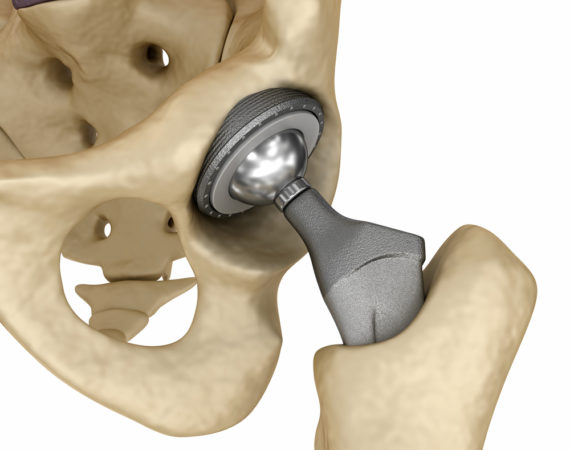
Johnson & Johnson to Pay $1 Billion in Pinnacle Hip Settlements
In a recent development in the multidistrict litigation (MDL) surrounding DuPuy Pinnacle hip products, Johnson & Johnson has agreed to pay $1 billion to resolve the majority of pending claims. Claimants have filed about 10,000 DuPuy Pinnacle hip lawsuits in the last several years over allegations of personal injury caused...
FDA Regulation of Medical Devices: How Safe are We?
The U.S. Food and Drug Administration (FDA) is a federal regulatory agency responsible for monitoring and reporting on food, pharmaceutical, and medical devices. A series of widespread injuries caused by FDA-approved drugs and medical devices - coupled with a damning scandal about their hidden database that distorts safety information -...
Boxed Warning Alert for Certain Insomnia Medications
The U.S. Food and Drug Administration (FDA) announced last week that certain insomnia medications will now feature a boxed warning. The FDA uses this type of warning, also known as a black box warning, on the label of prescription drugs to call attention to serious or life-threatening risks. The “black...
FDA Warns Consumers about Homeopathic Drugs
In April 2019, the U.S. Food and Drug Administration (FDA) published safety warning letters aimed at four companies who manufacture and market homeopathic drugs. Though homeopathic products do not need FDA approval to remain on the market, the federal agency still monitors and inspects the products to ensure compliance with...
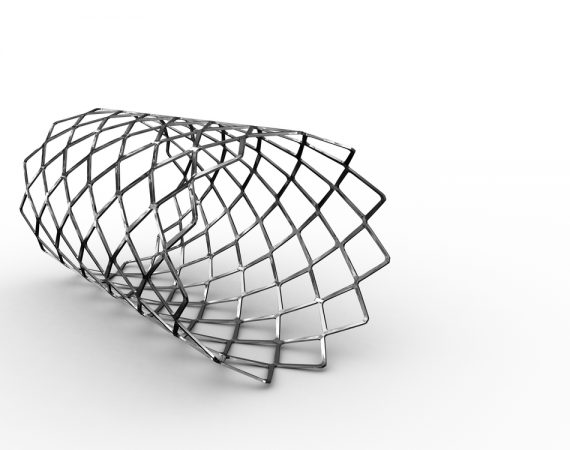
Device Safety Alert: FDA Warns Against Off-Label Use of Wingspan Stent
The Wingspan stent system manufactured by Stryker was recently the subject of a postmarket surveillance study. The results of the WEAVE study prompted an device safety alert from the U.S. Food and Drug Administration (FDA), which reminded healthcare providers about the dangers of using medical devices outside of their intended purpose....
Losartan Recall: More Blood Pressure Drugs Pulled from Shelves
Patients with high blood pressure can expect a more severe shortage of the medications they take to manage their condition. Last week, Torrent Pharmaceuticals Ltd. announced an expansion to the already gigantic losartan recall currently in place. Drug and Device Watch details the initial cause of the losartan recall in...
Defective Infant Product Linked to Numerous Infant Deaths
Fisher-Price recalled their Rock ‘n Play sleeper amid tragic reports of more than two dozen infant deaths associated with the defective infant product. In what many believe to be an attempt at avoiding a recall, the company initially issued a joint statement with the U.S. Consumer Product Safety Commission (CPSC)...
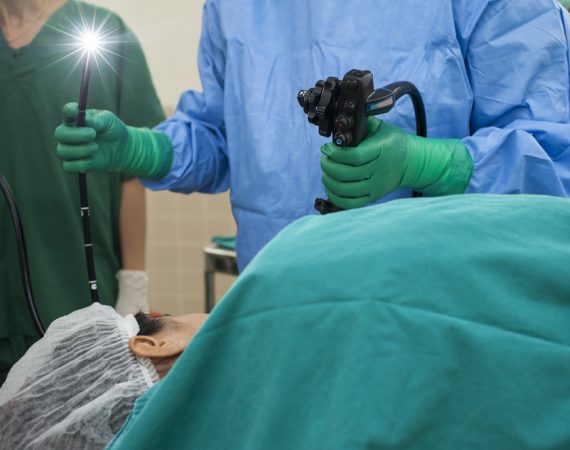
FDA: Medical Scope Infection Rates on the Rise
After years of improvement in the rate of medical scope infections, the U.S. Food and Drug Administration (FDA) recently began receiving reports of superbug infections associated with contaminated medical scopes. In 2015, the high rate of infections prompted the FDA to require medical scope manufacturers to conduct post-market studies on...